2.3.3 Testing for Halide Ions
- Dissolve halide ions solution in nitric acid and then add drops of silver nitrate.
- The nitric acid is to prevent any false positive results from carbonate ions precipitating out with silver ions
- Ag+ (aq) + X- (aq) → AgX (s)
- AgX would be a precipitate: silver chloride is white, silver bromide is cream, silver iodide is yellow.
- Add dilute then concentrated ammonia after as the colours are too similar.
- If the precipitate dissolves in dilute ammonia: chloride
- If the precipitate does not dissolve in dilute, but does in concentrated ammonia: bromide
- If the precipitate does not dissolve in either: iodide
3.1.12 Acids and bases
- Acid: proton donor
- Base: proton acceptor
- pH = –log10[H+ ]
- Water is slightly dissociated.
- Ionic product of water: Kw = [H+ ][OH–]
- Disocciation constant: pKa = –log10(Ka) because Ka can have a large range.
- Ka is larger for stronger acids as they disocciate completely in water.
- A buffer solution maintains an approximately constant pH, despite dilution or addition of small amounts of acid or base.
- E.g. if an acid dissociates, we have HX --> H+ + X-. If we add a base, it reacts with the H+ ions, so [X-] goes up and [H+] goes down by equal amounts. Ka and [HX] do not change.
- Acidic buffer solutions contain a weak acid and the salt of that weak acid.
- Basic buffer solutions contain a weak base and the salt of that weak base.
3.3.3 Halogenoalkanes
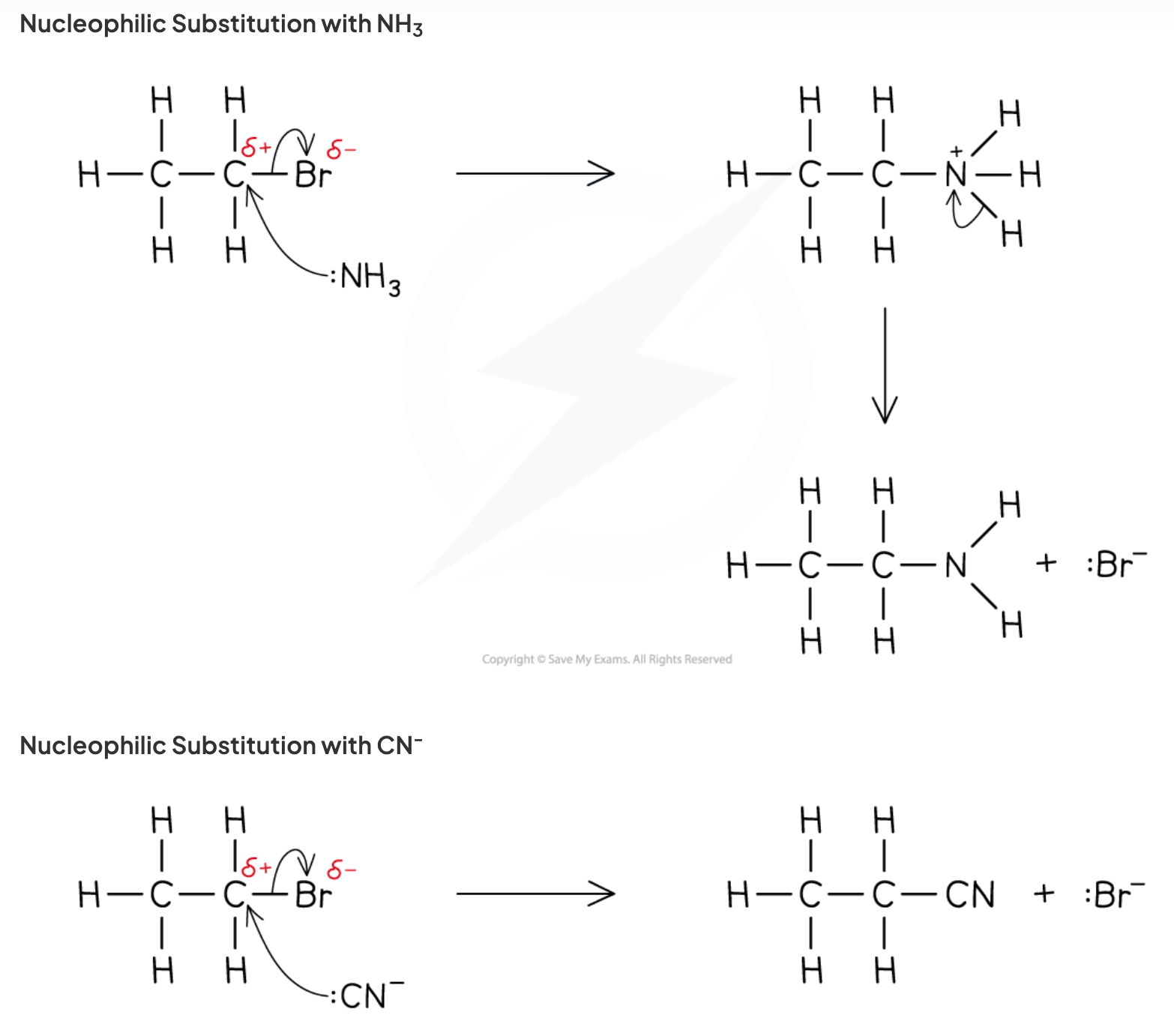
- Haloalkanes contain polar bonds as halogens are more electronegative than carbon.
- Nucleophiles like positive. :NH3, -:OH, CN:-
- Nucleophilic substitution: haloalkanes form alcohols or amines.
- Nucleophile attacks the δ+ carbon and the electrons are transferred to the halogen.
- The greater the Mr of the halogen in the polar bond, the easier it breaks e.g. CI has a weaker bond than CF.
- Nucleophilic substitution can only happen for 1o and 2o haloalkanes.
- Haloalkanes undergo elimination reactions at high temperatures under alcoholic conditions to form alkenes.
- The nucleophile accepts a proton so a hydrogen atom is removed from the haloalkane to form water.
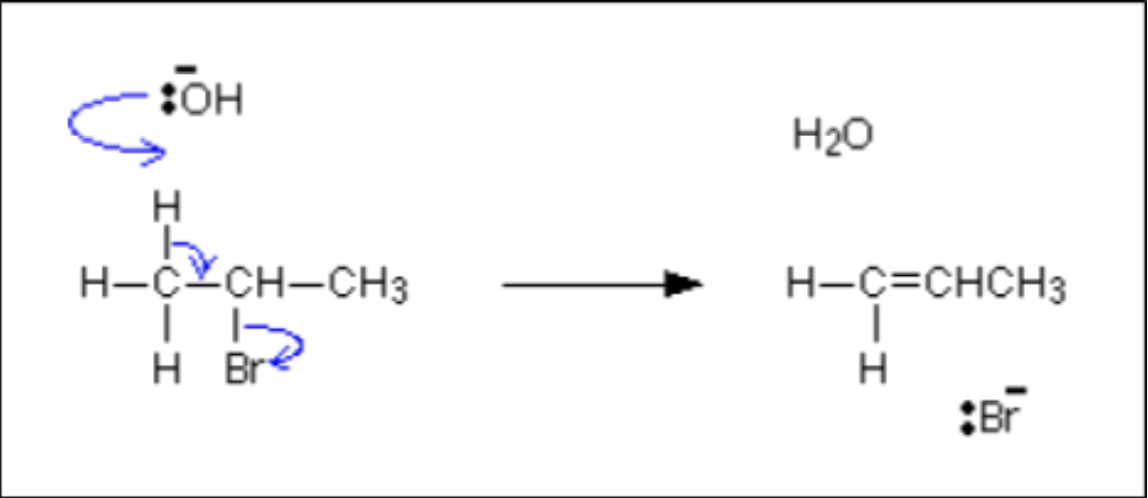
- Elimination only occurs with 2o and 3o haloalkanes.
- Different conditions will result in different reactions
- NaOH (hot, in ethanol): an elimination reaction occurs to form an alkene
- NaOH (warm, aqueous): a nucleophilic substitution reaction occurs, and an alcohol is formed
- Ozone and CFCs absorb UV light
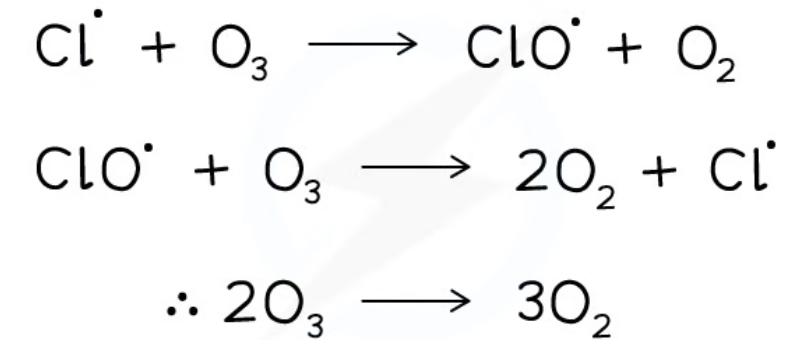
3.3.4 Alkenes
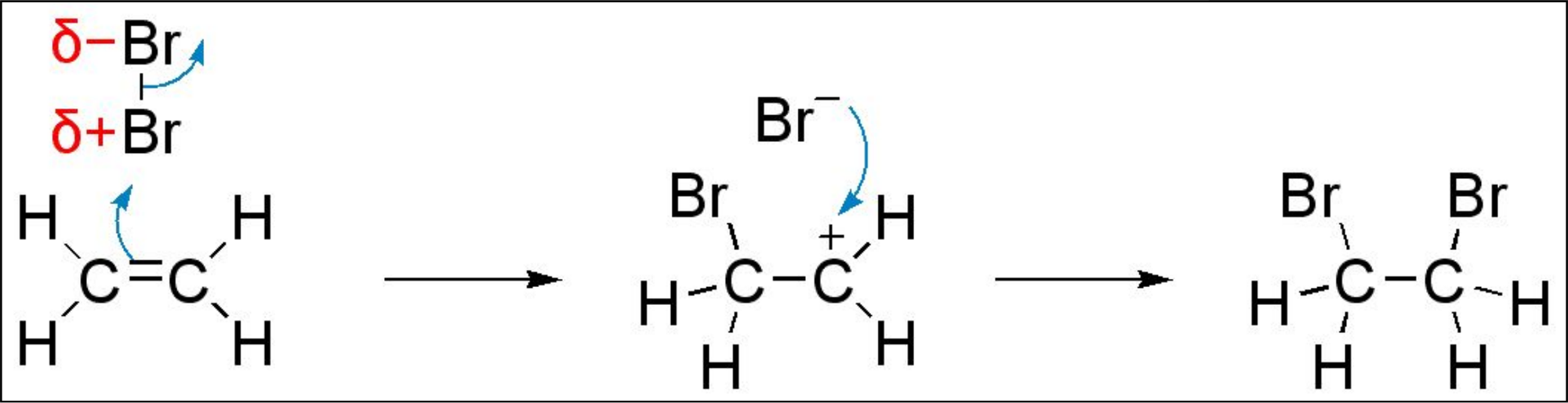
- Alkenes are unsaturated hydrocarbons with a double covalent bond, a centre of high electron density.
- Alkenes undergo electrophillic addition about the double bond.
- Double bond is broken. Carbocation (3 bonds) is formed. This has a positive charge. Tertiary = more stable = more likely to form.
- Electrophiles: electron acceptors. They are attracted to areas of high electron density e.g. the double bond.
- Common electrophiles: HBr, Br2, H2SO4.
- Products: alkyl hydrogensulfates or haloalkanes.
- Addition polymers: formed from alkenes. The double bond of the monomer breaks to form a repeat unit and many of these join together.
- Addition polymers are unreactive.
- Conditions: high pressure and temperature for branched chains with weak intermolecular forces, and vice versa.
- Uses: unreactive and non-biodegradable so plastics e.g. PVC.
3.3.5 Alcohols
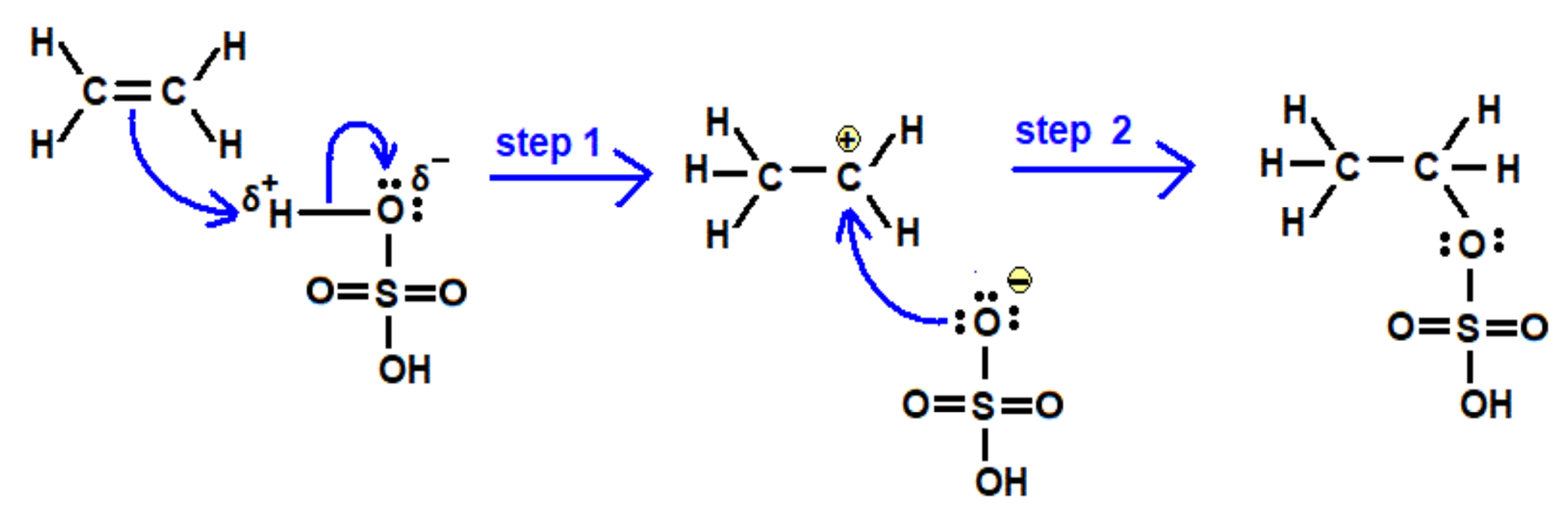
- Alcohols are produced by hydration of alkenes with an acid catalyst.
- Ethanol is produced industrially by fermentation of glucose.
- The conditions for this process. Ethanol produced industrially by fermentation is separated by fractional distillation and can then be used as a biofuel.
- Alcohols can be primary, secondary and tertiary. This is the number of alkyl groups the closest carbon is connected to.
- Primary alcohols can be oxidised to aldehydes which can be further oxidised to carboxylic acids.
- Secondary alcohols can be oxidised to ketones.
- Tertiary alcohols are not easily oxidised.
- Oxidising agent: acidified potassium dichromate(VI) and heat.
- Further oxidation: under reflux conditions.
- Alcohols can form alkenes from elimination reactions with acid catalysts.
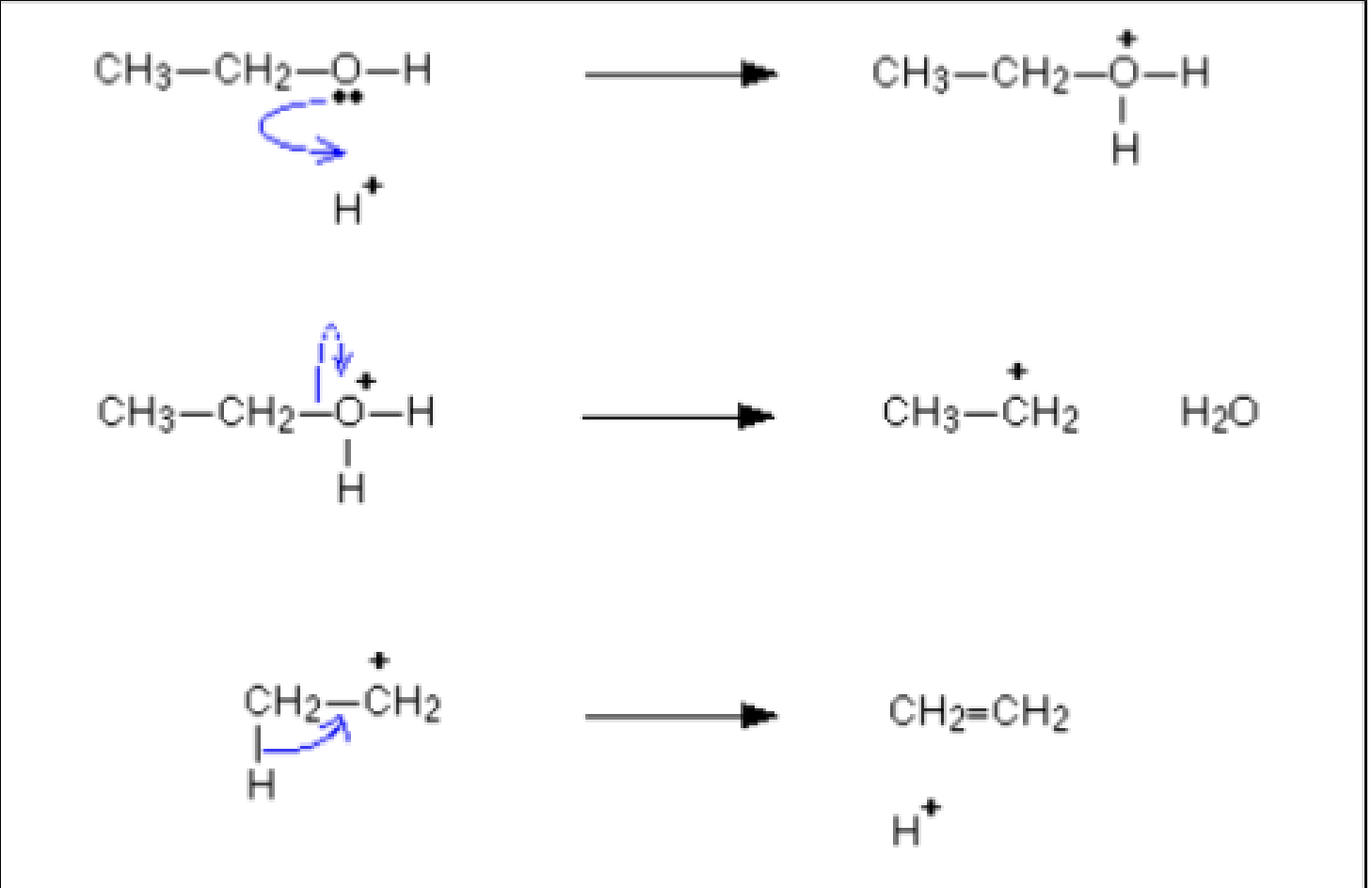
- These alkenes can produce addition polymers without using monomers from crude oil.
3,3.8 Aldehydes and Ketones
- Aldehydes are readily oxidised to carboxylic acids.
- Chemical tests to distinguish between aldehydes and ketones
- Fehling’s solution:
- Tollens’ reagent:
- Aldehydes can be reduced to primary alcohols, and ketones to secondary alcohols, using NaBH4 in aqueous solution. These reduction reactions are examples of nucleophilic addition.
- The nucleophilic addition reactions of carbonyl compounds with KCN, followed by dilute acid, to produce hydroxynitriles.
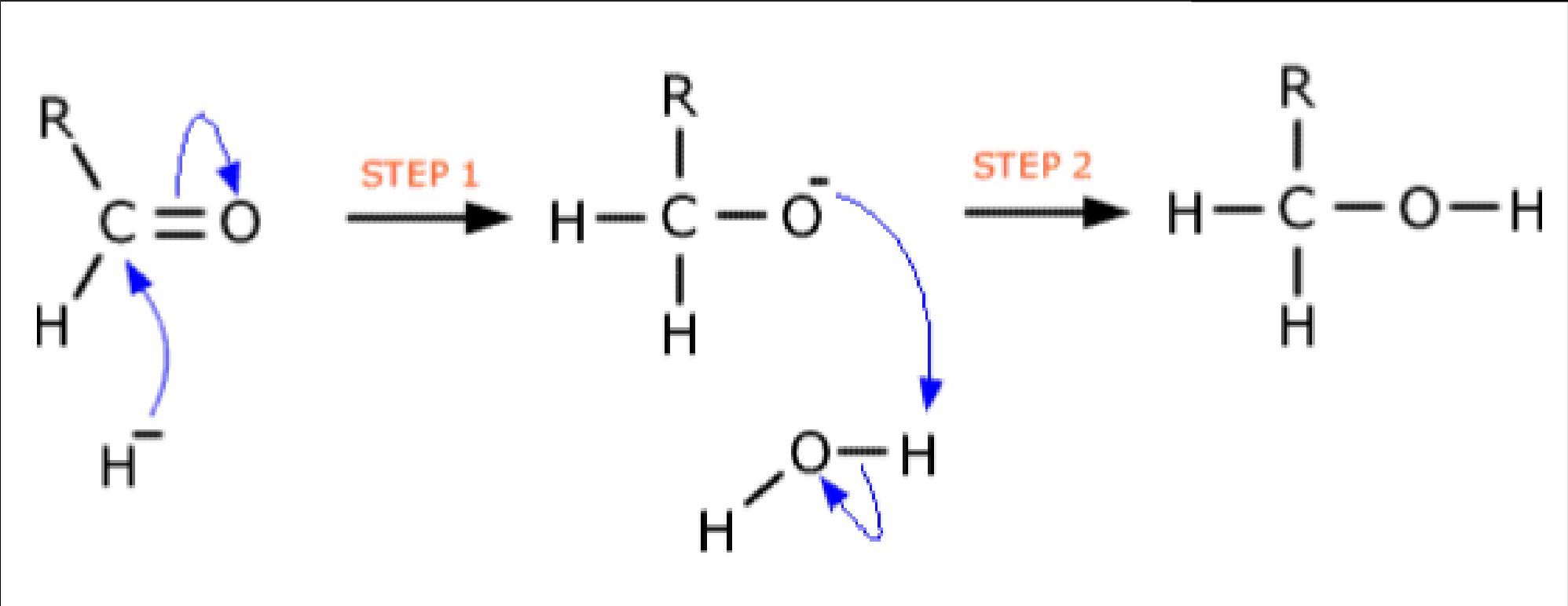
- The reducing agent: NaBH4 provides the nucleophile H-.
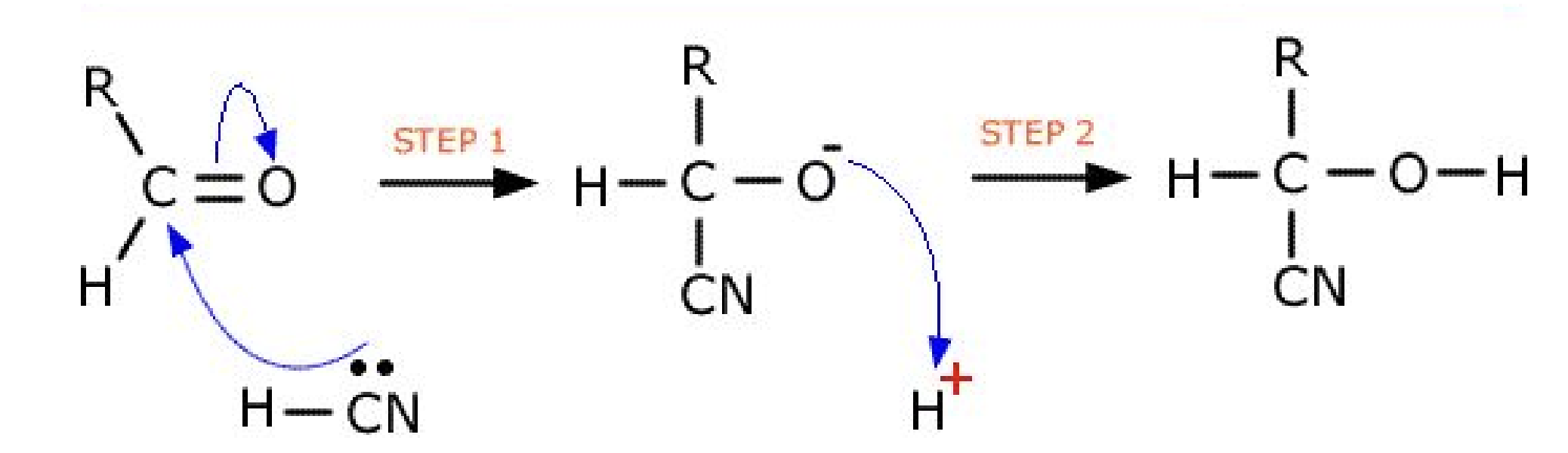
- Aldehydes and unsymmetrical ketones form mixtures of enantiomers when they react with KCN followed by dilute acid. The hazards of using KCN.
3.3.9 Carboxylic acids and Esters
- Carboxylic acids: COOH
- Carboxylic acids: weak acids, form COO- + H+.
- Esters: formed by reacting carboxylic acid + alcohol, in the presence of an acid catalyst under reflux. Water is a by-product.
- Esters: smell nice. Used as perfume, food flavouring.
- Vegetable oils: esters of glycerol (alcohol).
- Biodiesel: esters formed from vegetable oils and methanol with catalyst.
- Esters can be hydrolysed to form alcohols and carboxylic acids/ salts of carboxylic acids under acidic or alkaline conditions.
- If acidic conditions: alcobol and carboxyic acid.
- If alkaline conditions: alcobol and salt e.g. COO-Na+.
- Soap: Hydrolyise vegetable oils and animal fats in alkaline conditions.
3.3.10 Aromatic Chemistry
- Benzene is an aromatic compound containing six carbons and six hydrogens and a ring of delocalised electrons.
- Each bond has an intermediate length between a single and double bond.
- Benzene has a high melting point because of the delocalised outer electron from the p-orbital, but a low boiling point as they are non-polar (can't be dissolved in water).
- Benzene had an even lower enthalpy change (-208 kJ/mol) than predicted (-360 kJ/mol).
- Aromatic compounds undergo electrophillic substitution because of high electron density
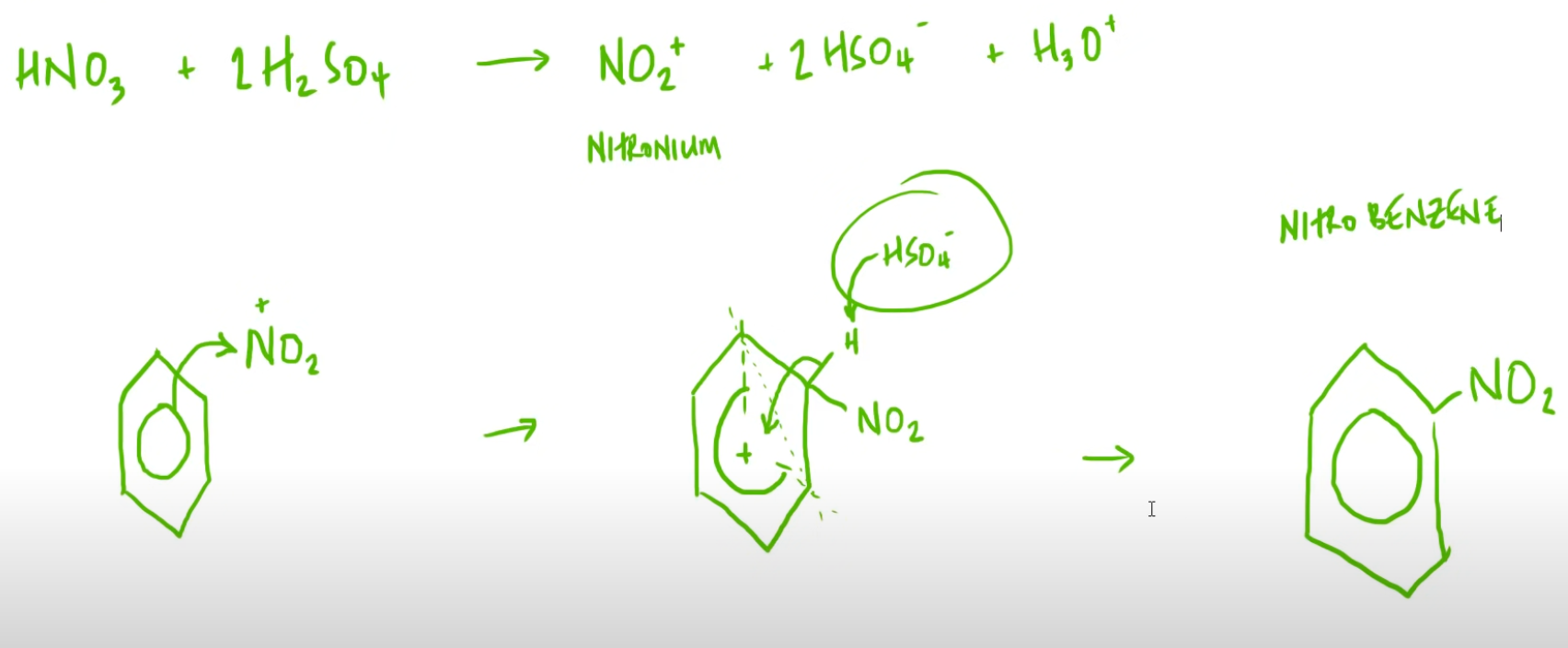
- This monosubstitution takes place at 55 oC.
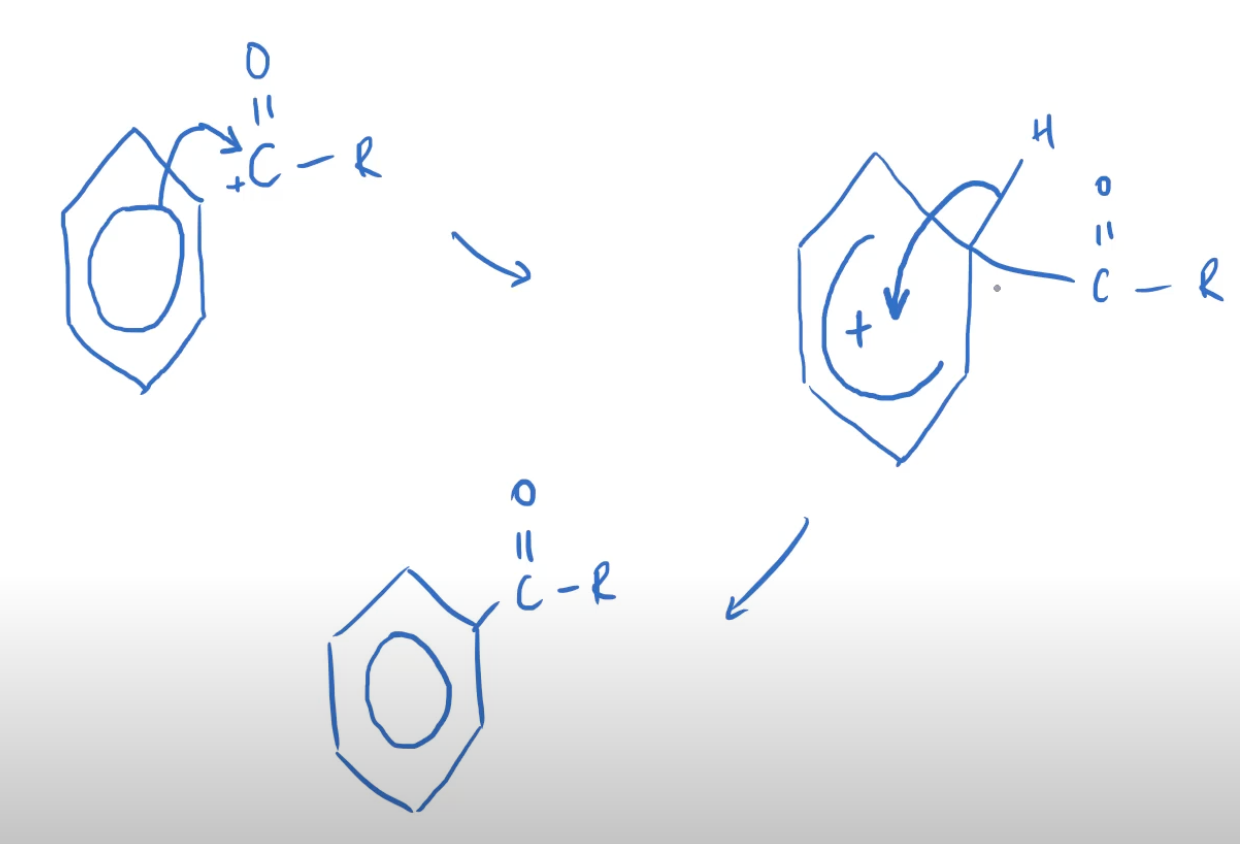
3.3.11 Amines
- Amines are formed when a H in ammonia is replaced with an R group.
- Primary amine: two H. Secondary amine: one H. Tertiary amine: no H.
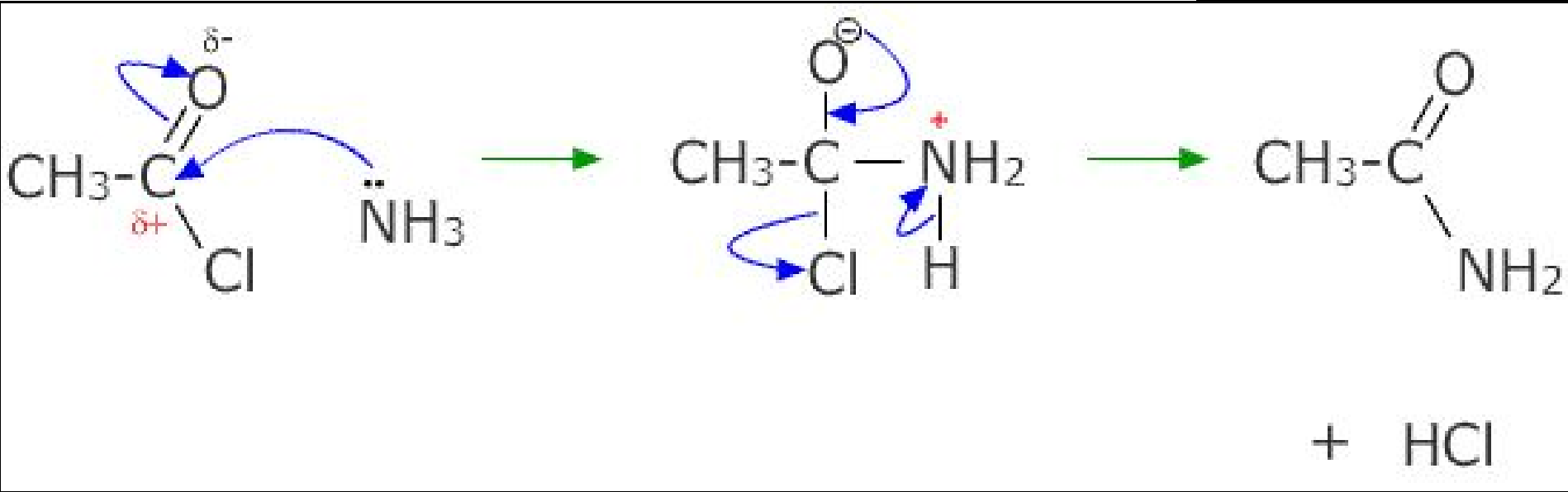
- To form an amine: nucleophilic substitution of a haloalkane with ammonia. Use excess ammonia to form a primary amine.
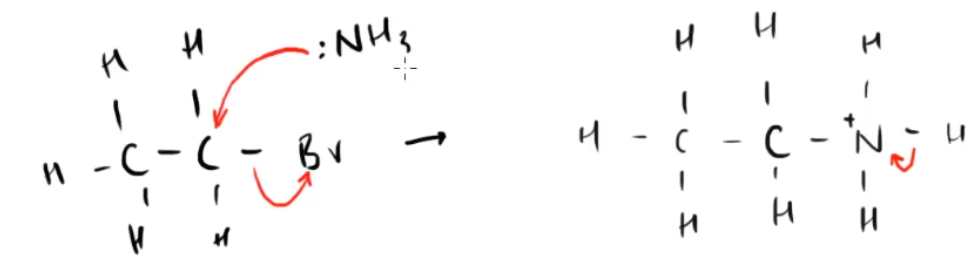
- To form a secondary amine, nucleophilic substitution of a primary amine with haloalkane.
- Another way to form amines: reduction of nitrile.
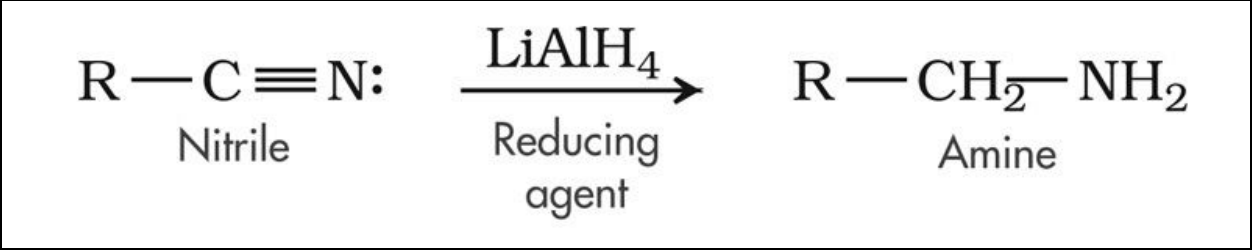
- Conditions of reduction: LiAlH4 and dilute acid OR hydrogen and nickel catalyst.
- Amines are weak bases. More electrons available, more basic.
- Amines undergo nucleophilic substitution as they are nucleophiles.
- quaternary ammonium saltsPosts
3.3.16 Chromatography
- Chromatography is used to separate mixtures.
- TLC (thin layer) is used to analyse small samples e.g. dyes.
- Stationary phase: thin metal sheet coated in alumina (Al2O3) or silica (SiO2)
- Different Rf values: each substance has different attraction to stationary and mobile phases.
- To identify the substances, use UV light.
- Sometimes we use two solvents because some of the substances do not separate/dissolve with the first/either solvent.
- Less polar = greater Rf value.
7.7.1 Amino Acids
- Amino acids have two functional groups (-NH2 and -COOH).
- 2-aminocarboxylic acids are naturally occuring amuino acods.

- General structural formula: RCH(NH2)COOH (there are 20 of these).
- Amino acids are amphoteric (acidic and basic).
- -COOH groups: act as acids
- -NH2 groups: act as bases
- -OH and -CONH2 groups: not able to act as acids or bases
- Amino acids will react with acids and bases.
- Zwitterions are formed when amino acids act within themselves (intramolecularly).
- A zwitterion is an ion with both a positive (-NH3+) and a negative (-COO-).
- Zwitterions have strong intermolecular forces because of the charges.
- Amino acids are therefore soluble crystalline solids.
- A solution of amino acids in water will exist as zwitterions with both acidic and basic properties, and act as buffer solutions.
- If an acid is added: -COO- forms -COOH (zwitterion becomes +ve).
- If a base is added: NH3+ forms the -NH2 group (zwitterion becomes -ve).
- The isoelectric point is a point when neither the negatively charged or positively charged ions dominate and the amino acid exists as a neutral zwitterion.
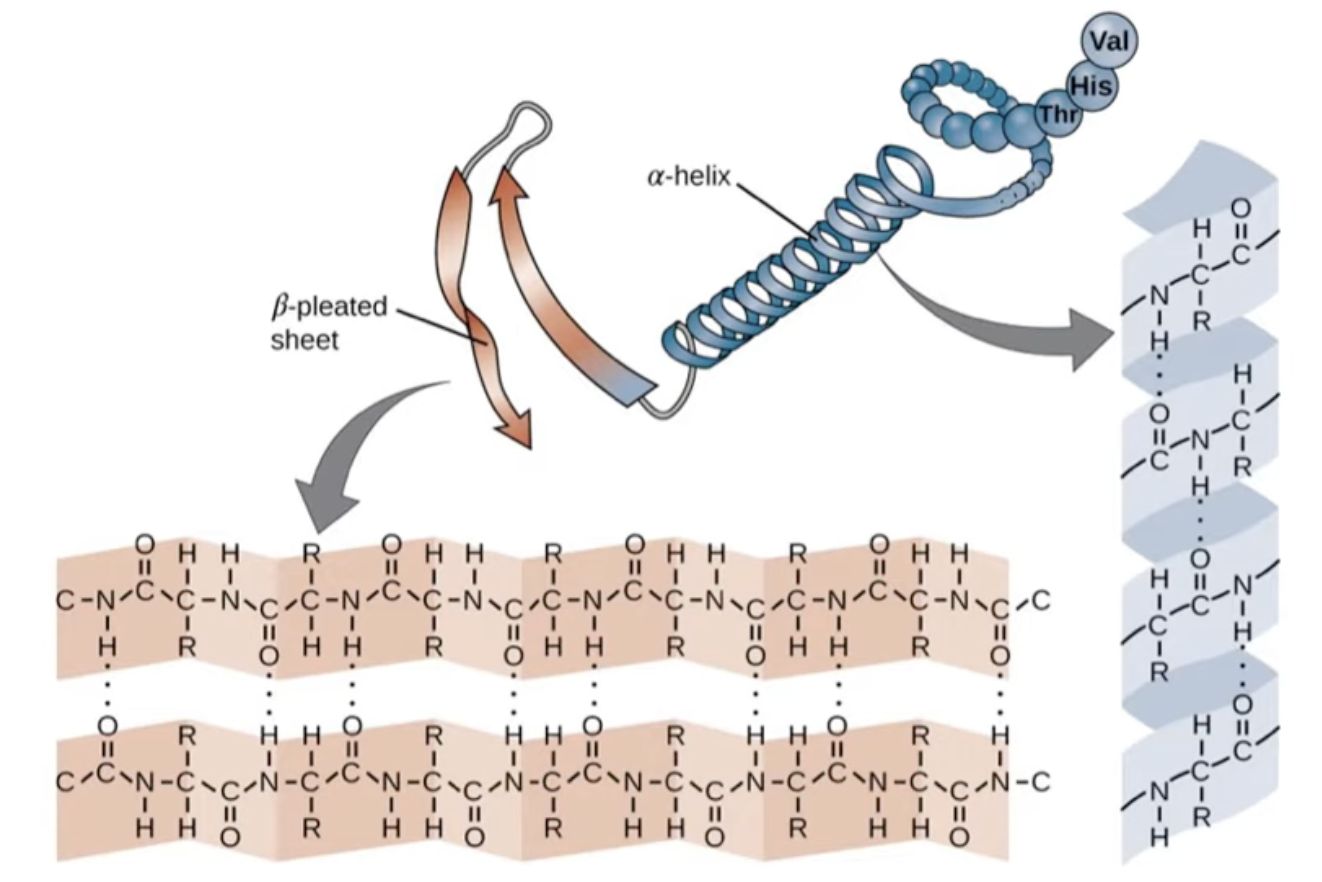
7.7.2 Proteins
- Dipeptides: the -NH2 group of one amino acid reacts with the -COOH group of another amino acid in a condensation reaction.
- It has the NH2 and the COOH groups at the ends, and a peptide bond (the H-N-C=O) in the middle.
- Polypeptide: formed when many amino acids join together to form a long chain of molecules.
- Protein
- Primary: many peptide covalent bonds
- Secondary: weakly negative N and O forming hydrogen bonds in a α-helix or β-pleated sheet
- Tertiary: between the R-groups
- weak hydrophobic
- disulphide bonds
- hydrogen bonds
- ionic bonds
- Hydrogen bonding occurs between the C=O groups and N-H groups
- Ionic attractions occur between the side chains of amino acids, for example between -COO- and -NH3+
- Cysteine is an amino acid that has a -CH2SH side group; two molecules can react together to make a sulfur-sulfur bridge between the two molecules
- Hydrolysis: the reversal of condensation. Break the peptide link and add H, H and O to form COOH and NH2 again.
- Hydrolysis conditions: chemically or using enzymes
- Reagent: concentrated hydrochloric acid. Boiled for hours, slow reaction.
- Enzyme: room temperature.
- You can identify the amino acid products using TLC.
- Amino acids can be located on a chromatogram using developing agents e.g. ninhydrin or ultraviolet light and identified by their Rf values.