- Energy is conserved in chemical reactions. The amount of energy in the universe at the end of a chemical reaction is the same as before the reaction takes place.
- Endothermic: energy is absorbed from the surroundings e.g. thermal decomposition.
- Exothermic: energy is released to the surroundings e.g. combustion.
- Activation Energy: minimum amount of energy that particles must have to react.
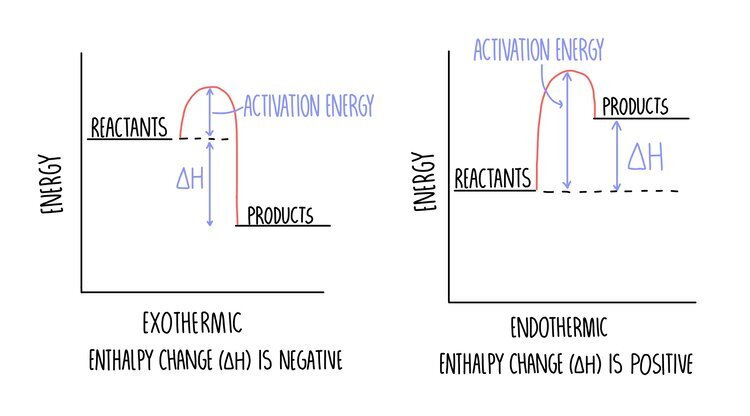
- Breaking reactant bonds is endothermic, forming product bonds is exothermic.
- Energy change = Reactants - Products (all the bonds)
Energy Changes (Topic 5) AQA GCSE Chemistry Notes for Triple Science Students Only
Next Update:
- Cells and Batteries
- Fuel Cells