Rate of Reaction and Equilibrium | AQA GCSE Chemistry | Topic 6
Last updated on
- Mean rate of reaction = amount of reactant used/ time taken = amount of product formed/ time taken.
- Rate of reaction can be measured in g/s or cm3/s etc.
- Rate of reaction is fastest at the start and then slows down.
- Exact rate of reaction: draw a tangent and work out gradient.
- Gradient = rise/ run between two points.
- Rate of reaction is fastest at the start and slowest at the end.
- Factors affecting rate of reaction: concentration, pressure, surface area of the reactants, temperature, catalysts.
- Apart from catalysts, they make particles closer together and increase frequency of collisions,
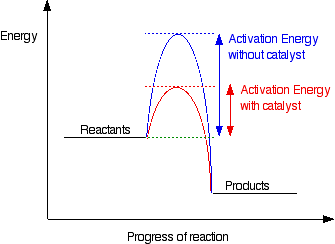
- Catalysts: provide an alternative reaction pathway with a lower activation energy, without being used up.
- Activation energy: minimum energy needed to start a reaction successfully.
 chemguide.co.uk
chemguide.co.uk- Reversible Reactions: when the products of the reaction can react to produce the original reactants
- Closed system: nothing can enter or leave the reactants
- Equilibrium: in a closed system, when the rate of forward reaction equals the rate of backward reaction
- Le Chatelier's Principle: when a condition is changed, equilibrium moves to oppose the change.
- Higher temperature: equilibrium moves to the endothermic side
- Lower temperature: equilibrium moves to the exothermic side
- Higher concentration of reactants: equilibrium moves to the right
- Lower concentration of reactants: equilibrium moves to the left
- Higher concentration of products: equilibrium moves to the left
- Lower concentration of products: equilibrium moves to the right
- Higher pressure: equilibrium moves to the side with less moles of gas (count big numbers and count gas only).
- Lower pressure: equilibrium moves to the side with more moles of gas
