Definitions
- exothermic reactions
- energy is released to the surroundings
- endothermic
- energy is absorbed from the surroundings
- activation energy
- minimum energy needed to start a reaction
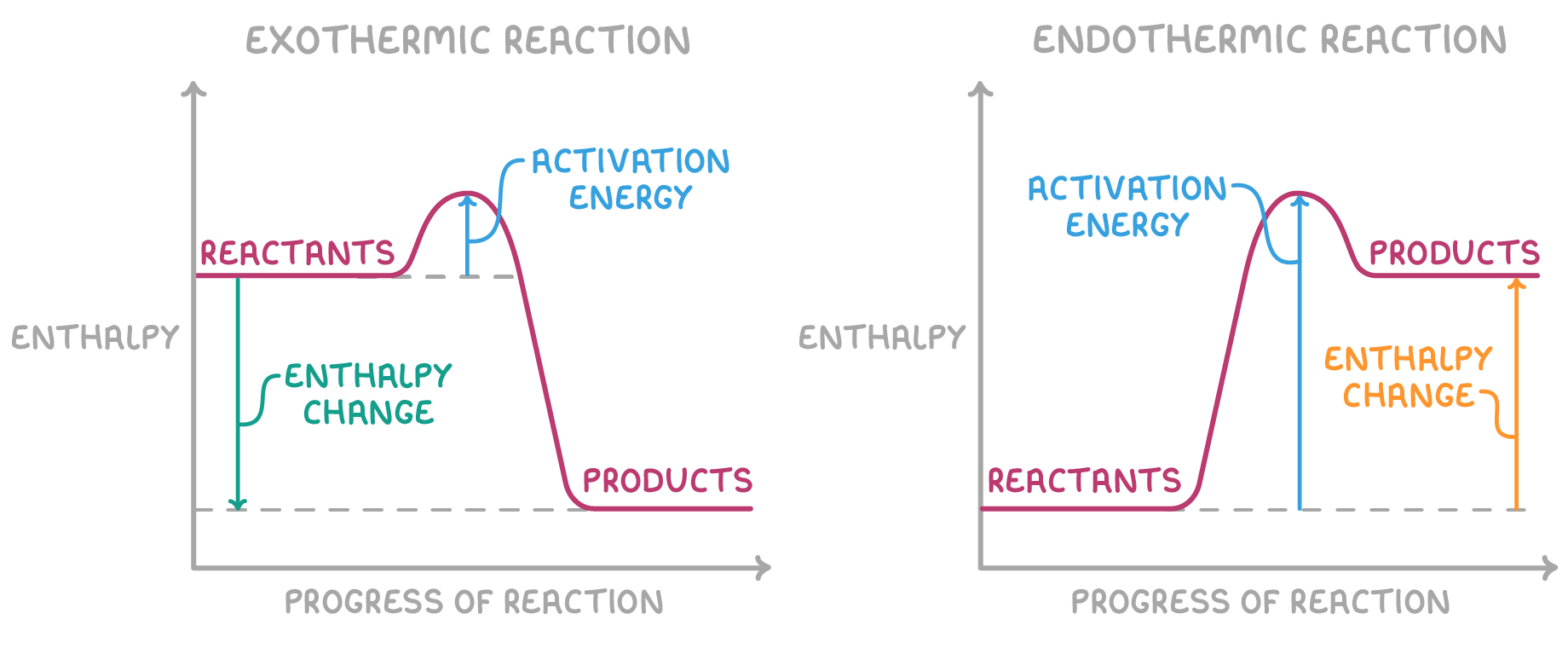
Energy profile diagrams
- x-axis
- progress of reaction
- y-axis
- energy
- diagram
- reactants
- products
- energy change - from reactants to products
- activation energy - from reactants to peak
Calculating bond energy
- add up all the energy needed to break the reactant bonds
- add up all the energy released from forming the product bonds
- energy change = reactants total - products total