Le Chatelier's Principle
- if the conditions of a dynamic equilibrium are changed, the equilibrium will move to counteract the change
Dynamic equilibrium
- rate of forward reaction = rate of backward reaction
- in a reversible reaction
- in a closed system
- no substances are either added to the system or lost from it
Examples - which way does equilibrium move?
- an increase in temperature - endothermic direction
- an increase in pressure - side with fewer molecules of gas
- a decrease in temperature - exothermic direction
- a decrease in pressure - side with more molecules of gas
- an increase in concentration of reactants - right
- an increase in concentration of products - left
- a decrease in concentration of reactants - left
- a decrease in concentration of products - right
Haber process
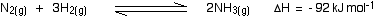
- Rate of reaction - faster
- high pressure
- high concentration
- high temperature
- Equilibrium - shift to the right
- low temperature - to shift equilibrium in exothermic direction
- high pressure - to shift to side with fewer moles of gas
- Overall compromise
- high pressure - although expensive - 200 atm
- medium/ low temperature - 400o C
- iron catalyst