1) What mass of hydrogen is produced when 192 g of magnesium is reacted with hydrochloric acid?
Mg + 2 HCl → MgCl2 + H2 (3 marks)
2) What mass of oxygen is needed to react with 8.5 g of hydrogen sulphide (H2S)?
2 H2S + 3 O2 → 2 SO2 + 2 H2O (3 marks)
3) What mass of potassium oxide is formed when 7.8 g of potassium is burned in oxygen?
4 K + O2 → 2 K2O (3 marks)
4) Railway lines are welded together by the Thermitt reaction, which produces molten iron. What mass of iron is formed from 1 kg of iron oxide?
Fe2O3 + 2 Al → 2 Fe + Al2O3 (3 marks)
5) What mass of oxygen is required to oxidise 10 g of ammonia to NO?
4 NH3 + 5 O2 → 4 NO + 6 H2O (3 marks)
6) What mass of aluminium oxide is produced when 135 g of aluminium is burned in oxygen?
2 Al + 3 O2 → Al2O3 (3 marks)
Limiting Reactant
Question 1

Question 2
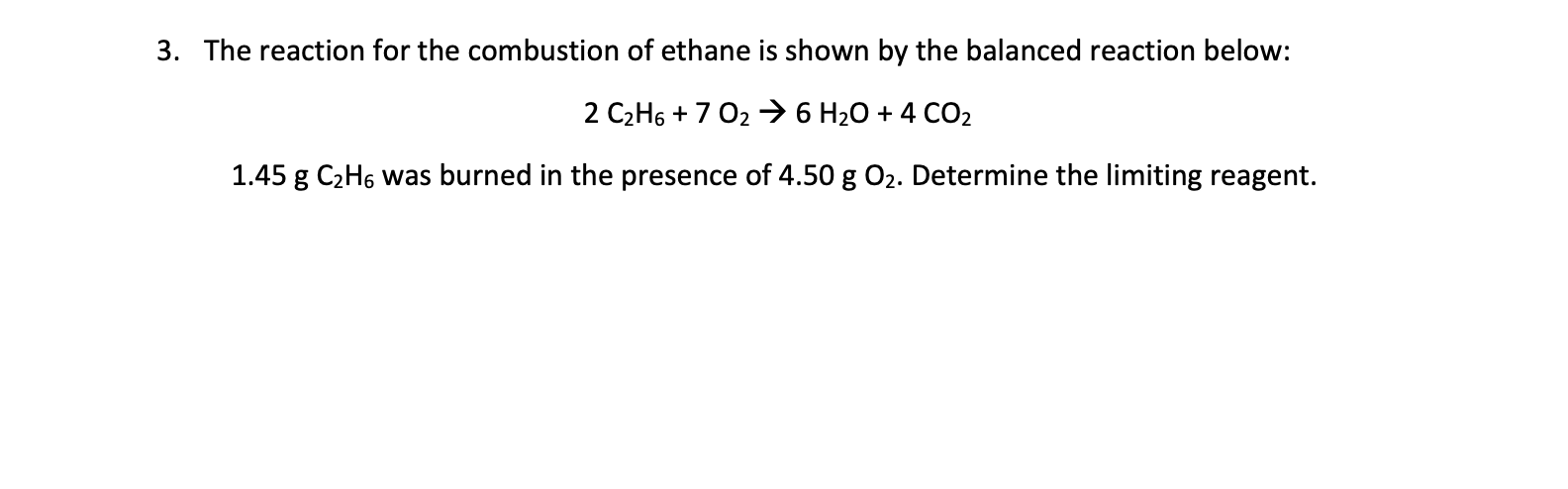
Question 3

Question 4
